Now Available
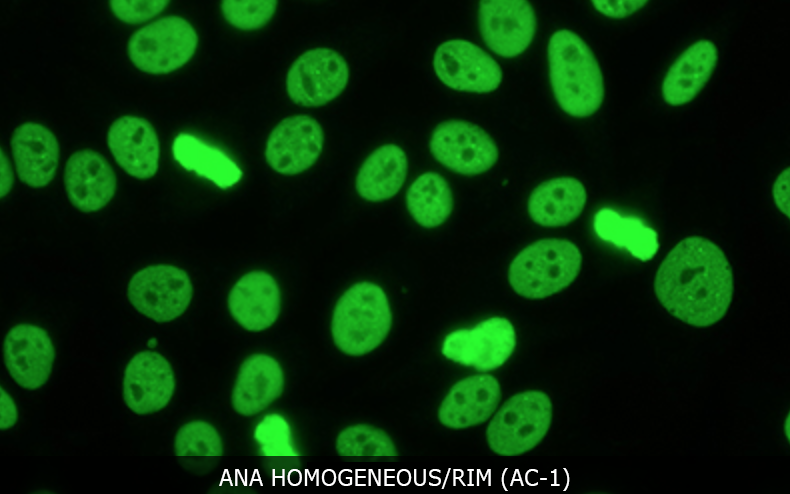
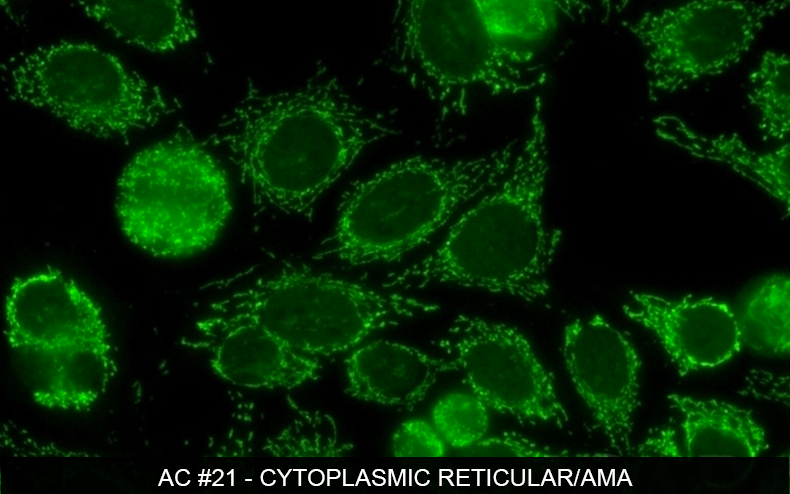
Antinuclear Antibodies(ANA) IUIS Reference Standards

A message from the Autoantibody Standardization Committee
(ASC, www.AutoAb.org)
One of the goals of the IUIS Autoantibody Standardization Committee (ASC) is to help laboratories improve standardization and quality control of testing of anti-nuclear and related autoantibodies. As part of this effort, the ASC, in cooperation with the Centers for Disease Control (CDC) USA, is making reference standards available for autoantibody specificities through PSG. In addition to the same 17 reference reagents previously available from CDC, new reference reagents will be generated and distributed by PSG. Two new references are the anti-mitochondrial and anti-rods/rings autoantibodies – for details, please see publication (Calise SJ, Zheng B, Hasegawa T, Satoh M, Isailovic N, Ceribelli A, Andrade LEC, Boylan K, Cavazzana I, Fritzler MJ, Garcia de la Torre I, Hiepe F, Kohl K, Selmi C, Shoenfeld Y, Tincani A, Chan EKL. Reference standards for the detection of anti-mitochondrial and anti-rods/rings autoantibodies. Clin Chem Lab Med. 2018:in press. Reprint request send to SCalise@dental.ufl.edu or echan@ufl.edu).
*Availability is limited to 1 vial of each ANA reference per laboratory.
To request reagents, please download and fill out our Reference Reagent Order Request Form. Choose either the (PDF Version) or the (Word Doc Version)
and forward or email to referencereagents@plasmaservicesgroup.com
Please CC: echan@ufl.edu
PDF Order Form or choose Word Doc Order Form
Please visit www.ANApatterns.org for detailed descriptions and images of a variety of ANA patterns.
Visit ANApatterns.org
Please visit the Autoantibody Standardization Committee at www.AutoAb.org for more detailed information.
