PSG proudly announces the launch of our new, donor-centered website.
Please visit the site to view our innovative donor video, updated FAQs -, new donor form -, current paid studies, and “PSG + You page”.
Read MoreA Medical and Research Diagnostics Company.
Interested in donating? Sign up online.
Eligible donors are compensated $200 for every future donation at our site!
IUIS Autoantibody Standardization Committee, ASC & CDC Reference standards for autoantibody specificities through PSG. See below for more information.
What can we do for you?
Why use Plasma Services Group?
Plasma Services Group prides itself on being a leader in the specialty antibody collection process for the medical diagnostic and pharmaceutical industries.
We have built our company around our Quality Policy Statement: “We at Plasma Services Group are committed to understanding our suppliers’ and customers’ individual requirements and meeting their expectations for service and support in a cost-effective manner.” This is why we are a vital and valued part of the IVD industry.
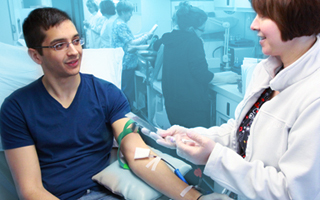
Why Donate? You will be monetarily compensated for your time and rewarded with the knowledge that you are providing a critical component of the diagnostic process for patients with a similar condition. Our donors are always provided with a safe, private and comfortable donation process.

We offer bulk disease state plasma collected and/or processed to meet your needs.
Contact us to see what we can do for you!
Learn More
A message from the Autoantibody Standardization Committee (ASC, www.AutoAb.org)
One of the goals of the IUIS Autoantibody Standardization Committee (ASC) is to help laboratories improve standardization and quality control of testing of anti-nuclear and related autoantibodies. As part of this effort, the ASC, in cooperation with the Centers for Disease Control (CDC) USA, is making reference standards available for autoantibody specificities through PSG. In addition to the same 17 reference reagents previously available from CDC, new reference reagents will be generated and distributed by PSG. Two new references are the anti-mitochondrial and anti-rods/rings autoantibodies – for details, please see publication (Calise SJ, Zheng B, Hasegawa T, Satoh M, Isailovic N, Ceribelli A, Andrade LEC, Boylan K, Cavazzana I, Fritzler MJ, Garcia de la Torre I, Hiepe F, Kohl K, Selmi C, Shoenfeld Y, Tincani A, Chan EKL. Reference standards for the detection of anti-mitochondrial and anti-rods/rings autoantibodies. Clin Chem Lab Med. 2018:in press. Reprint request send to SCalise@dental.ufl.edu or echan@ufl.edu).
*Availability is limited to 1 vial of each ANA reference per laboratory.
To request reagents, please download and fill out our Reference Reagent Order Request Form. Choose either the (PDF Version) or the (Word Doc Version) and forward or email to referencereagents@plasmaservicesgroup.com
Please CC: echan@ufl.edu
PDF Order Form or choose Word Docx Order Form
Please visit www.ANApatterns.org for detailed descriptions and images of a variety of ANA patterns.
Visit ANApatterns.org
Please visit the Autoantibody Standardization Committee at www.AutoAb.org for more detailed information.

Please visit the site to view our innovative donor video, updated FAQs -, new donor form -, current paid studies, and “PSG + You page”.
Read More
PSG volunteered at our 1st Multiple Myeloma Walk in Hollywood, Florida on March 10th!
Read More
Meet The PSG Team at the 11th International Congress on Autoimmunity.
Read More
JOIN THE GLOBAL LABORATORY MEDICINE COMMUNITY
The 70th AACC Annual Scientific Meeting & Clinical Lab Expo

The Association of Medical Laboratory Immunologists, 31st Annual Scientific Meeting, JW Marriott Scottsdale Camelback Inn, Resort & Spa – Scottsdale, Arizona
Read More
The world’s leading trade fair for the medical industry invites you to Düsseldorf from 12 – 15 November 2018. Be part of the No. 1!
Read More
Kathryn Kohl joins PSG as Director of Scientific and Clinical Affairs, bringing with her over 25 years of medical device and project development expertise.
Read More